Coronavirus: age cap in Oxford vaccine trials casts doubt over results
AstraZeneca failed to disclose that under-55s made up test group in which jab was most effective

Oxford University and AstraZeneca are facing questions over their promising vaccine trial results after health officials revealed that key information about the ages of the test subjects was omitted.
During the Phase 3 trial, one group of test subjects was inoculated with two doses of the vaccine, spaced a month apart, while a second group was administered with a half-dose followed by a full one. In the first group, the protection rate was found to be 62%, but data from the second group showed that the vaccine was effective 90% of the time.
The reason for the wide discrepency has puzzled scientists. But they may now have their answer, after researcher Moncef Slaoui, who is spearheading the US’s vaccine distribution programme, told reporters that the age of participants in the second group “was capped at 55”, Bloomberg reports.
Subscribe to The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
No age breakdown was given in the reporting of the results, which have been cast into doubt over the omission of the most at-risk age demographics.
Former Pfizer research and development president Geoffrey Porges researchers “said he thought it was unlikely the AstraZeneca jab would get approval in the US after the company ‘tried to embellish their results’ by highlighting higher efficacy in a ‘relatively small subset of subjects in the study’”, the Financial Times reports.
Meanwhile, David Salisbury, an associate fellow of the global health programme at Chatham House, told The Telegraph: “You’ve taken two studies for which different doses were used and come up with a composite that doesn’t represent either of the doses. I think many people are having trouble with that.”
And the admission earlier this week by AstraZeneca that the half-dose was administered by error during the trials will add to “questions about whether the vaccine’s apparently spectacular efficacy will hold up under additional testing”, says The New York Times - which suggests that AstraZeneca’s “spotty disclosures have eroded confidence” in the jab.
Create an account with the same email registered to your subscription to unlock access.
Sign up for Today's Best Articles in your inbox
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
-
 5 high-caliber cartoons about Kristi Noem shooting her puppy
5 high-caliber cartoons about Kristi Noem shooting her puppyCartoons Artists take on the rainbow bridge, a farm upstate, and more
By The Week US Published
-
 The Week Unwrapped: Why is the world running low on blood?
The Week Unwrapped: Why is the world running low on blood?Podcast Scientists believe universal donor blood is within reach – plus, the row over an immersive D-Day simulation, and an Ozempic faux pas
By The Week Staff Published
-
 Rishi Sunak's asylum spat with Ireland explained
Rishi Sunak's asylum spat with Ireland explainedIn Depth Irish government plans to override court ruling that the UK is unsafe for asylum seekers
By The Week UK Published
-
 Covid four years on: have we got over the pandemic?
Covid four years on: have we got over the pandemic?Today's Big Question Brits suffering from both lockdown nostalgia and collective trauma that refuses to go away
By Chas Newkey-Burden, The Week UK Published
-
 The hollow classroom
The hollow classroomOpinion Remote school let kids down. It will take much more than extra tutoring for kids to recover.
By Mark Gimein Published
-
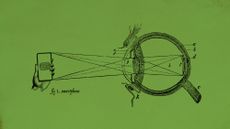 Excess screen time is making children only see what is in front of them
Excess screen time is making children only see what is in front of themUnder the radar The future is looking blurry. And very nearsighted.
By Devika Rao, The Week US Published
-
 Covid-19: what to know about UK's new Juno and Pirola variants
Covid-19: what to know about UK's new Juno and Pirola variantsin depth Rapidly spreading new JN.1 strain is 'yet another reminder that the pandemic is far from over'
By Arion McNicoll, The Week UK Published
-
 Long-term respiratory illness is here to stay
Long-term respiratory illness is here to stayThe Explainer Covid is not the only disease with a long version
By Devika Rao, The Week US Published
-
 Covid inquiry: the most important questions for Boris Johnson
Covid inquiry: the most important questions for Boris JohnsonTalking Point Former PM has faced weeks of heavy criticism from former colleagues at the public hearing
By The Week Staff Published
-
 China's pneumonia cases: should we be worried?
China's pneumonia cases: should we be worried?The Explainer Experts warn against pushing 'pandemic panic button' following outbreak of respiratory illness
By Keumars Afifi-Sabet, The Week UK Published
-
 Vallance diaries: Boris Johnson 'bamboozled' by Covid science
Vallance diaries: Boris Johnson 'bamboozled' by Covid scienceSpeed Read Then PM struggled to get his head around key terms and stats, chief scientific advisor claims
By The Week UK Published