Faulty medical implants: who is at risk?
New investigation shows patients worldwide are being harmed as a result of poor regulation and lax testing rules
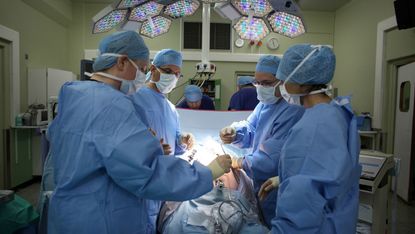
Faulty medical implants have caused the deaths of more than 1,000 people in just three years, a global investigation has found.
Between 2015 and 2018, UK regulators alone received 62,000 “adverse incident” reports linked to components including pacemakers, artificial hips, breast implants and contraceptives.
A third of the incidents “had serious repercussions for the patient, and 1,004 resulted in death”, reports The Guardian.
Subscribe to The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The new research into the dangers associated with the global $400bn (£310bn) industry was conducted by the International Consortium of Investigative Journalists (ICIJ), and involved more than 250 reporters in 36 countries.
The investigation “found a lax system of regulation in Europe that allows companies to ‘shop around’ dozens of safety organisations until one of them approves their product”, says the BBC.
It also found that “doctors can be left in the dark about the true risk of treatments they are recommending to their patients”, the news site adds.
Who has been affected?
The Guardian says that examples of failure in the implant market include “replacement hips and vaginal mesh products sold to hospitals without any clinical trials; patients relying on faulty pacemakers when manufacturers were aware of problems; complications with hernia mesh that ruled one of Britain’s top athletes out of competing for years; and regulators approving spinal disc replacements that later disintegrated and migrated in patients”.
Among the many other instances, patients were given birth control implants that caused internal damage and bleeding; mesh implants for incontinence caused abdominal pain in some patients; and implanted defibrillators were also reported to misfire.
A panel of experts put together by the ICIJ is advising anyone with implants who may be concerned to seek help, adding: “Your first point of call should be the medical team that performed the operation. If you cannot go back to them for whatever reason, you should consult your primary care doctor.
“The doctor should be able to refer you to a specialist who is familiar with the device and the surgery you had.”
What is the medical community saying?
The British Royal College of Surgeons (RCS) is calling for “drastic regulatory changes”. RSC president Professor Derek Alderson said: “All implantable devices should be registered and tracked to monitor efficacy and patient safety in the long-term.”
Meanwhile, MedTech Europe, the body that represents the medical devices industry, said: “Millions of people have safely benefited from medical devices and can now live healthier, more productive and more independent lives.
“Life is unimaginable today without the hundreds of thousands of medical devices in our hospitals and in our homes.”
Create an account with the same email registered to your subscription to unlock access.
Sign up for Today's Best Articles in your inbox
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
-
 'Good riddance to the televised presidential debate'
'Good riddance to the televised presidential debate'Instant Opinion Opinion, comment and editorials of the day
By Harold Maass, The Week US Published
-
 Caitlin Clark the No. 1 pick in bullish WNBA Draft
Caitlin Clark the No. 1 pick in bullish WNBA DraftSpeed Read As expected, she went to the Indiana Fever
By Peter Weber, The Week US Published
-
 Today's political cartoons - April 16, 2024
Today's political cartoons - April 16, 2024Cartoons Tuesday's cartoons - sleepyhead, little people, and more
By The Week US Published
-
 Dengue hits the Americas hard and early
Dengue hits the Americas hard and earlySpeed Read Puerto Rico has declared an epidemic as dengue cases surge
By Peter Weber, The Week US Published
-
 How happy is Finland really?
How happy is Finland really?Today's Big Question Nordic nation tops global happiness survey for seventh year in a row with 'focus on contentment over joy'
By Harriet Marsden, The Week UK Published
-
 How Tehran became the world's nose job capital
How Tehran became the world's nose job capitalUnder the radar Iranian doctors raise alarm over low costs, weak regulation and online influence of 'Western beauty standards'
By Harriet Marsden, The Week UK Published
-
 Martha's Rule: patients given right to urgent second opinion
Martha's Rule: patients given right to urgent second opinionThe Explainer Hospitals in England will launch new scheme that will allow access to a rapid treatment review
By Richard Windsor, The Week UK Published
-
 Africa's renewed battle against female genital mutilation
Africa's renewed battle against female genital mutilationUnder the radar Campaigners call for ban in Sierra Leone after deaths of three girls as coast-to-coast convoy prepares to depart
By Harriet Marsden, The Week UK Published
-
 The contaminated blood scandal
The contaminated blood scandalThe Explainer Widely regarded as the worst treatment disaster in the history of the NHS, the public inquiry is due to publish its report in May
By The Week UK Published
-
 Can Britain's dental crisis be fixed?
Can Britain's dental crisis be fixed?The Explainer New proposals include more money for dentists working in under-served areas
By Richard Windsor, The Week UK Published
-
 Argentina: the therapy capital of the world
Argentina: the therapy capital of the worldUnder the radar Buenos Aires natives go hungry to pay for psychoanalysis, amid growing instability, anxiety – and societal acceptance
By Harriet Marsden, The Week UK Published