Valneva vaccine: why UK ditched jab with ‘stronger immune response’ than AstraZeneca
Downing Street accused French manufacturer of breaching contract
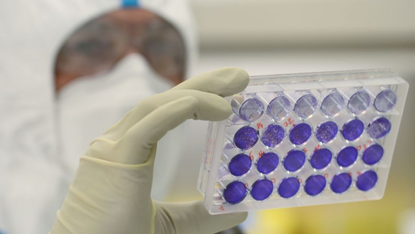
Shares in the French biotech company Valneva soared yesterday after a trial showed that its Covid-19 vaccine “demonstrated superiority” over the AstraZeneca jab. The results were published less than a month after the UK government terminated a contract to buy 100 million doses from the French firm.
The Valneva vaccine had been set to play a significant part in the UK’s ongoing vaccine rollout. In August last year, Downing Street announced a multimillion-pound investment in Valneva’s manufacturing plant in Livingston, Scotland, with Business Secretary Kwasi Kwarteng touting it as a possible “vaccine production powerhouse”.
In February, the government increased its order of 60 million jabs, securing an extra 40 million doses of the “promising vaccine candidate”, for 2021 and 2022. But the deal came crashing down last month when Downing Street served notice that the UK was to terminate the agreement.
Subscribe to The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The government claimed Valneva was in breach of the deal because the jab had not been approved by the UK regulator in time to “form any part of our vaccine rollout in autumn and winter”. The biotech company “strenuously denies” the allegation, and has continued the vaccine’s development, said the BBC.
This week, a phase 3 clinical trial of 4,012 adults found that Valneva’s VLA2001 jab produced more neutralising antibodies in the recipient than AstraZeneca’s vaccine. Participants experienced “far fewer side effects” and displayed a “stronger immune response” than those who had received the AZ jab, said the Financial Times.
The trial’s chief investigator, Professor Adam Finn, described the immune response as “both impressive and extremely encouraging”. The vaccine has been developed with a more “traditional approach” to others being used in Europe and North America at present, he explained, making it the only “whole virus, inactivated, adjuvanted vaccine” being tested in Europe. The results suggest that the jab is “on track to play an important role in overcoming the pandemic”, said Finn.
Competition for Covid-19 vaccines has “proved politically explosive” on a global front, noted the FT. The EU only last month settled an “acrimonious dispute” with AstraZeneca over delayed supplies, and the French government initially came in for criticism last year when the UK first struck a supply deal with Valneva, said the paper.
The UK has so far approved four vaccines for use: Pfizer-BioNTech, Oxford-AstraZeneca, Moderna and Janssen. However, under-40s have been offered an alternative to the AstraZeneca vaccine due to evidence linking it to rare blood clots.
Shares in Valneva fell by 42% when the UK scrapped its deal in September, but they shot up again yesterday by 32% premarket with the publication of the trial results. While the company said it would still work to gain approval from the UK’s medical regulator, the company “may now look to other countries” for future deals, said the BBC. The government has described the Valneva agreement as an “ongoing commercial issue”.
If approved, the jab could prove beneficial to countries in the developing world as it is “based on traditional vaccine technology”, said The Guardian. This could help to combat low vaccination rates and the expense of investing in new technologies to develop newer mRNA vaccines, like the Moderna and Pfizer jabs.
The Guardian added that the Valneva vaccine could be useful in “countries where ultra-low-temperature storage is hard to come by” as it can be stored in normal refrigerators.
Create an account with the same email registered to your subscription to unlock access.
Sign up for Today's Best Articles in your inbox
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Julia O'Driscoll is the engagement editor. She covers UK and world news, as well as writing lifestyle and travel features. She regularly appears on “The Week Unwrapped” podcast, and hosted The Week's short-form documentary podcast, “The Overview”. Julia was previously the content and social media editor at sustainability consultancy Eco-Age, where she interviewed prominent voices in sustainable fashion and climate movements. She has a master's in liberal arts from Bristol University, and spent a year studying at Charles University in Prague.
-
 Who actually needs life insurance?
Who actually needs life insurance?The Explainer If you have kids or are worried about passing on debt, the added security may be worth it
By Becca Stanek, The Week US Published
-
 Sexual wellness trends to know, from products and therapies to retreats and hotels
Sexual wellness trends to know, from products and therapies to retreats and hotelsThe Week Recommends Talking about pleasure and sexual health is becoming less taboo
By Theara Coleman, The Week US Published
-
 Is the AI bubble deflating?
Is the AI bubble deflating?Today's Big Question Growing skepticism and high costs prompt reconsideration
By Joel Mathis, The Week US Published
-
 What are the rules on cutting sick pay for unvaccinated staff?
What are the rules on cutting sick pay for unvaccinated staff?feature Ikea joins growing list of firms axing sick pay entitlement for employees who haven’t had Covid jabs
By The Week Staff Published
-
 A year in the City: from ruinous shortages to creeping inflation
A year in the City: from ruinous shortages to creeping inflationBusiness Briefing 2021 was a year of supply chain disruptions and big stock market floats
By The Week Staff Published
-
 The Bank of England official warning women against home working
The Bank of England official warning women against home workingWhy Everyone’s Talking About Not returning to the office will result in ‘two track’ career development, senior policymaker claims
By The Week Staff Published
-
 End of the furlough scheme: what happens next for the UK’s job market?
End of the furlough scheme: what happens next for the UK’s job market?Business Briefing 1.6m workers were still being supported by the scheme in July
By The Week Staff Published
-
 UK travel industry ‘choked’ by Covid restrictions
UK travel industry ‘choked’ by Covid restrictionsfeature Seven in ten firms planning redundancies as holiday bookings collapse
By The Week Staff Published
-
 Leaked government report reveals what post-Freedom Day restrictions could cost UK
Leaked government report reveals what post-Freedom Day restrictions could cost UKUnder the Radar England set to drop face masks and social distancing from 19 July
By The Week Staff Published
-
 Freedom Day delay: ‘catastrophic’ for businesses on life support
Freedom Day delay: ‘catastrophic’ for businesses on life supportfeature Billions to be wiped from economy and Sunak rejects calls to extend furlough scheme
By Mike Starling Published
-
 ‘The man who saved the world’: is AstraZeneca’s boss worth his millions?
‘The man who saved the world’: is AstraZeneca’s boss worth his millions?Today's Big Question Pascal Soriot’s bonus might be deserved, but that doesn’t mean it’s necessarily wise
By The Week Staff Published