The promise of longer-lasting immunity: ‘variant-proof’ Covid vaccine begins trials
On-trial jab may offer better protection against new strains of coronavirus
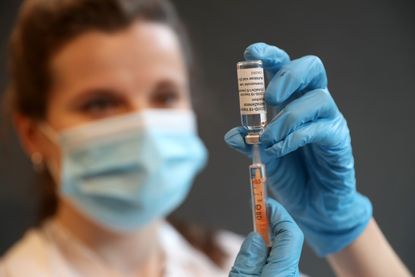
Trials for a “variant-proof” booster vaccine have begun in Manchester, raising hopes that the elderly and vulnerable could achieve long-lasting immunity against Covid-19.
Bolton-based couple Andrew and Helen Clarke, aged 63 and 64 respectively, were the first trial participants to receive the mRNA vaccine, which scientists hope will do away with the need to tweak jabs regularly to combat new strains, The Telegraph reported.
The results of the phase-one trial, which was launched by US pharmaceutical company Gritstone in collaboration with the University of Manchester and the Manchester University NHS Foundation Trust, are expected by the spring.
Subscribe to The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
Most Covid vaccines target the coronavirus’s spike protein – a small grappling hook on the outside of the virus that it uses to latch onto human cells. However, new variants have spike protein mutations, which can make current vaccines less effective.
The new vaccine, currently called GRT-R910, contains other viral proteins that are less likely to evolve over time, and is also designed to induce a strong memory T-cell response.
The University of Manchester’s Professor Andrew Ustianowski, chief investigator for the study, said that “the immune response to first generation vaccines can wane, particularly in older people” and “we think GRT-R910 as a booster vaccination will elicit strong, durable, and broad immune responses”.
Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, who is not involved in the trial, told The Guardian that “using parts of the virus that are less prone to change in virus variants has the potential to provoke an immune response that will be more effective against all current virus variants and any future variants that might develop”.
Sign up for Today's Best Articles in your inbox
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
The trial will be broadened to test the jab’s efficacy beyond the elderly and in other vulnerable populations. It will examine dose, safety, tolerability, and immunogenicity at least four months after the second dose of an initial vaccine.
The Clarkes said they had agreed to volunteer for the trial to “play their part” in ending the pandemic.
Andrew Clarke added: “Somebody has to be the first and we’re confident in the science and technology behind this vaccine and convinced of the need for it. We feel that this is perhaps a small part we can play in helping to make things change.”
Create an account with the same email registered to your subscription to unlock access.
-
 Nigeria's worsening rate of maternal mortality
Nigeria's worsening rate of maternal mortalityUnder the radar Economic crisis is making hospitals unaffordable, with women increasingly not receiving the care they need
By Harriet Marsden, The Week UK Published
-
 'Elevating Earth Day into a national holiday is not radical — it's practical'
'Elevating Earth Day into a national holiday is not radical — it's practical'Instant Opinion Opinion, comment and editorials of the day
By Harold Maass, The Week US Published
-
 UAW scores historic win in South at VW plant
UAW scores historic win in South at VW plantSpeed Read Volkswagen workers in Tennessee have voted to join the United Auto Workers union
By Peter Weber, The Week US Published
-
 Covid four years on: have we got over the pandemic?
Covid four years on: have we got over the pandemic?Today's Big Question Brits suffering from both lockdown nostalgia and collective trauma that refuses to go away
By Chas Newkey-Burden, The Week UK Published
-
 The hollow classroom
The hollow classroomOpinion Remote school let kids down. It will take much more than extra tutoring for kids to recover.
By Mark Gimein Published
-
 Excess screen time is making children only see what is in front of them
Excess screen time is making children only see what is in front of themUnder the radar The future is looking blurry. And very nearsighted.
By Devika Rao, The Week US Published
-
 Covid-19: what to know about UK's new Juno and Pirola variants
Covid-19: what to know about UK's new Juno and Pirola variantsin depth Rapidly spreading new JN.1 strain is 'yet another reminder that the pandemic is far from over'
By Arion McNicoll, The Week UK Published
-
 Long-term respiratory illness is here to stay
Long-term respiratory illness is here to stayThe Explainer Covid is not the only disease with a long version
By Devika Rao, The Week US Published
-
 Covid inquiry: the most important questions for Boris Johnson
Covid inquiry: the most important questions for Boris JohnsonTalking Point Former PM has faced weeks of heavy criticism from former colleagues at the public hearing
By The Week Staff Published
-
 China's pneumonia cases: should we be worried?
China's pneumonia cases: should we be worried?The Explainer Experts warn against pushing 'pandemic panic button' following outbreak of respiratory illness
By Keumars Afifi-Sabet, The Week UK Published
-
 Vallance diaries: Boris Johnson 'bamboozled' by Covid science
Vallance diaries: Boris Johnson 'bamboozled' by Covid scienceSpeed Read Then PM struggled to get his head around key terms and stats, chief scientific advisor claims
By The Week UK Published