Why your AstraZeneca vaccine may mean European holidays are off-limits
Month-long wait for Indian-made jabs to be approved on the continent
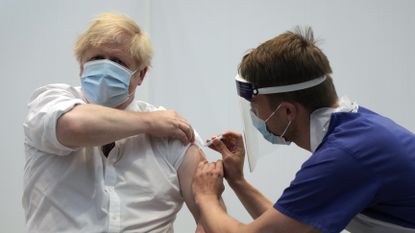
Brits given doses of the Oxford-AstraZeneca vaccine made in India face a month-long wait to be allowed to travel into almost half of Europe.
In a letter seen by The Telegraph, the Serum Institute of India (SII), one of the world’s largest vaccine manufacturers, said it may take weeks to resolve the “politics” that are causing British holidaymakers to be turned away from flights to Europe.
Sent to a recipient of the vaccine, the letter stated: “Sadly, this is out of our hands – we are doing our best to expedite this and it is up to the countries really to accept our product as official vaccine certificates are not issued by us. This is a bureaucratic matter and political matter at the country level.”
Subscribe to The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
The SII also stressed that the vaccine was “identical” to the Oxford-AstraZeneca jabs manufactured in the UK, noting that “15 countries in Europe have already approved Covishield and these batches, the rest should be concluded within a month”.
Millions of people in the UK were inoculated using doses manufactured by SII which are yet to be recognised by the European Medicines Agency (EMA). Unless the vaccines are given market approval by the EMA, thousands of Britons could be turned away at EU border crossings when their vaccine batch numbers are checked digitally.
The EU Digital Covid Certificate launched last Thursday does not recognise the version of the AstraZeneca vaccine known locally as Covishield and manufactured by SII.
“Up to five million doses of the version of the AstraZeneca jab in question have been administered in the UK”, ITV reports. They are recognisable by the vaccine batch numbers 4120Z001, 4120Z002, 4120Z003 which are written on the vaccine cards handed out after a jab is administered and are also available on the NHS app.
Sign up for Today's Best Articles in your inbox
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
The Department of Health has refused to confirm exactly how many India-developed AstraZeneca jabs have been administered in the UK. However, five million doses were imported in March this year. Authorities in the UK have used the name Vaxzevria on all UK medical records where the AstraZeneca vaccine has been administered.
“As we continue to cautiously reopen international travel, NHS Covid Pass will be a key service that allows people to demonstrate their Covid-19 vaccination status,” a spokesperson for the department said. “All AstraZeneca vaccines given in the UK are the same product and appear on the NHS Covid Pass as Vaxzevria,” they added.
The Telegraph tracked down three Britons affected, none of whom were told in advance they were to receive the SII-developed version of the AstraZeneca vaccine.
“Quite frankly [I feel] discriminated against, for lack of a better word,” 21-year-old Hannah Smith, who found that her first dose of the AstraZeneca vaccine was produced in India when she checked the batch numbers, told the paper.
Another person who wished to remain anonymous added: “That vaccine passports would be a thing is entirely predictable, so our government should have made sure any they purchased would be recognised for travel everywhere.”
The decision to bar people inoculated with the SII-manufactured vaccines has created “friction” between the EU and India, Reuters reports.
The exclusion of the jabs, produced using “analogous methods to the EU-approved AstraZeneca vaccine”, has prompted “anger and the threat of retaliatory measures by India against travellers from Europe”.
Last week, Indian Foreign Minister Subrahmanyam Jaishankar tweeted that he “took up Covishield authorisation for travel to Europe” during a meeting with the Vice-President of the European Commission Josep Borrell Fontelles.
“There is no suggestion that the Indian manufactured doses are in any way substandard,” The Telegraph says, noting that the vaccine does not have EU approval because “Indian manufacturers have not yet sought a licence for the product in Europe” as “the SII intend to predominantly supply low and middle income countries”.
Create an account with the same email registered to your subscription to unlock access.
-
 In what countries is assisted dying legal or under review?
In what countries is assisted dying legal or under review?In the spotlight More countries are granting more people the right to die
By Devika Rao, The Week US Published
-
 Nigeria's worsening rate of maternal mortality
Nigeria's worsening rate of maternal mortalityUnder the radar Economic crisis is making hospitals unaffordable, with women increasingly not receiving the care they need
By Harriet Marsden, The Week UK Published
-
 Dengue hits the Americas hard and early
Dengue hits the Americas hard and earlySpeed Read Puerto Rico has declared an epidemic as dengue cases surge
By Peter Weber, The Week US Published
-
 Covid four years on: have we got over the pandemic?
Covid four years on: have we got over the pandemic?Today's Big Question Brits suffering from both lockdown nostalgia and collective trauma that refuses to go away
By Chas Newkey-Burden, The Week UK Published
-
 How happy is Finland really?
How happy is Finland really?Today's Big Question Nordic nation tops global happiness survey for seventh year in a row with 'focus on contentment over joy'
By Harriet Marsden, The Week UK Published
-
 The hollow classroom
The hollow classroomOpinion Remote school let kids down. It will take much more than extra tutoring for kids to recover.
By Mark Gimein Published
-
 How Tehran became the world's nose job capital
How Tehran became the world's nose job capitalUnder the radar Iranian doctors raise alarm over low costs, weak regulation and online influence of 'Western beauty standards'
By Harriet Marsden, The Week UK Published
-
 Africa's renewed battle against female genital mutilation
Africa's renewed battle against female genital mutilationUnder the radar Campaigners call for ban in Sierra Leone after deaths of three girls as coast-to-coast convoy prepares to depart
By Harriet Marsden, The Week UK Published

